Phase Fraction as a Function of Temperature
Purpose: Learn to calculate and use a phase fractions vs. temperature plot
Module: PanPhaseDiagram
Thermodynamic Database: AlMgZn.tdb
Batch file: Example_#1.3.pbfx
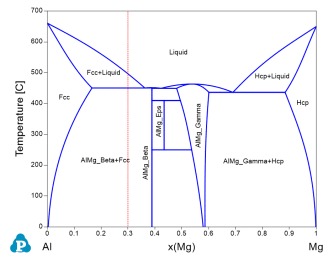
In Phase Fraction as a Function of Composition, we have calculated the fraction of each phase as a function of composition. In most cases, people would like to know phase transformation when temperature varies. In this example, we calculate the fraction of phases as a function of temperature for an AlMg binary alloy with composition of x(Mg)=0.3 (the red dash line in Figure 1). Such a calculation is especially valuable for a multi-component alloy.
Calculation Procedures:
-
Load AlMgZn.tdb following the procedure in Pandat User's Guide: Load Database, and select Al and Mg two components;
-
Perform 1D calculation following the procedure in Pandat User's Guide: Line Calculation (1D);
-
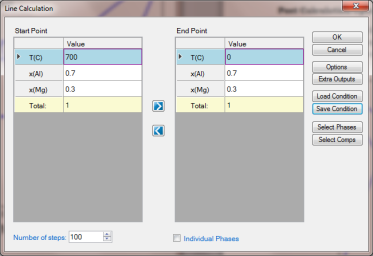
Set Calculation Condition as shown in Figure 2;
Post Calculation Operation:
-
Add legend for graph following the procedure in Pandat User's Guide: Icons for Graph on Toolbar;
-
Change graph appearance following the procedure in Pandat User's Guide: Property;
Information obtained from this calculation:
-
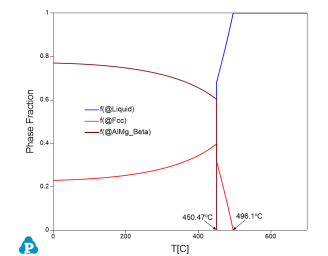
Liquid is the only stable phase at high temperature until 496.1 °C, which is the liquidus temperature;
-
Below liquidus, Fcc phase forms and its fraction increases while that of the Liquid phase decreases until the eutectic temperature 450.47 °C;
-
At eutectic temperature 450.47 °C, Liquid is disappeared (drops to 0% from 68.3%), fraction of the AlMg_Beta phase jumps from 0% to 60.21% and that of the Fcc phase jumps from 31.7% to 39.79%;
-
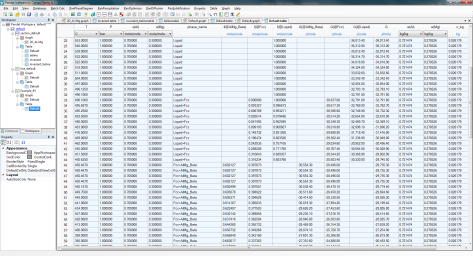
Details on the fraction of each phase as a function of temperature can also be found in the Default table as shown in Figure 4;